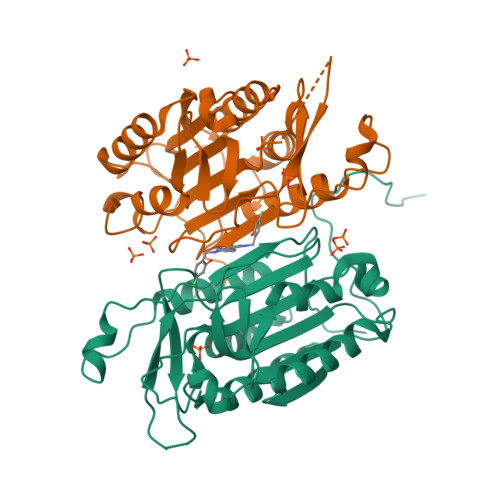
Tailoring small molecules for an allosteric site on procaspase-6.
Murray, J., Giannetti, A.M., Steffek, M., Gibbons, P., Hearn, B.R., Cohen, F., Tam, C., Pozniak, C., Bravo, B., Lewcock, J., Jaishankar, P., Ly, C.Q., Zhao, X., Tang, Y., Chugha, P., Arkin, M.R., Flygare, J., Renslo, A.R.(2014) ChemMedChem 9: 73-77
- PubMed: 24259468
- DOI: https://doi.org/10.1002/cmdc.201300424
- Primary Citation of Related Structures:
4N5D, 4N6G, 4N7J, 4N7M, 4NBK, 4NBL, 4NBN - PubMed Abstract:
Although they represent attractive therapeutic targets, caspases have so far proven recalcitrant to the development of drugs targeting the active site. Allosteric modulation of caspase activity is an alternate strategy that potentially avoids the need for anionic and electrophilic functionality present in most active-site inhibitors. Caspase-6 has been implicated in neurodegenerative disease, including Huntington's and Alzheimer's diseases. Herein we describe a fragment-based lead discovery effort focused on caspase-6 in its active and zymogen forms. Fragments were identified for procaspase-6 using surface plasmon resonance methods and subsequently shown by X-ray crystallography to bind a putative allosteric site at the dimer interface. A fragment-merging strategy was employed to produce nanomolar-affinity ligands that contact residues in the L2 loop at the dimer interface, significantly stabilizing procaspase-6. Because rearrangement of the L2 loop is required for caspase-6 activation, our results suggest a strategy for the allosteric control of caspase activation with drug-like small molecules.
Organizational Affiliation:
Departments of Structural Biology, Biochemical Pharmacology, Neuroscience, and Discovery Chemistry, Genentech, Inc., 1 DNA Way, South San Francisco, CA 94080 (USA). murray.jeremy@gene.com.